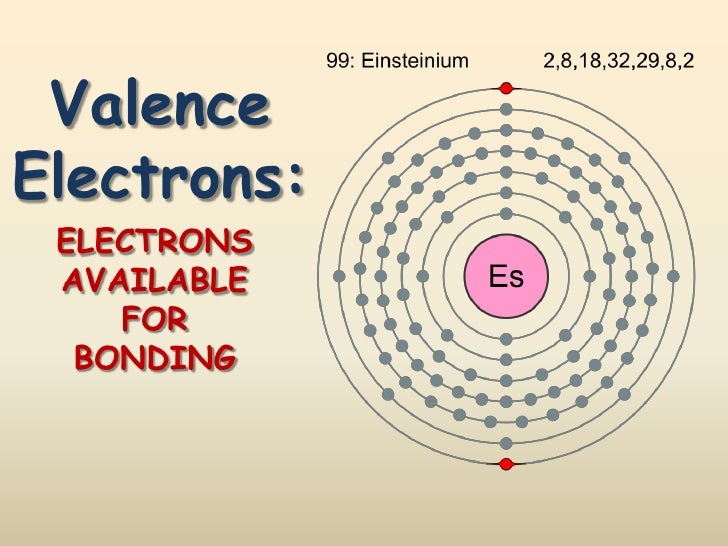
Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms. Because valence electrons are so important, atoms may be represented. The Group 1 8 atoms have 8 valence electrons with the exception of Helium which has 2 electrons The transition metals are more difficult to determine the number of valence electrons. Some of their valence electrons are in the inner shells.
Did you ever play the card game called go fish? Players try to form groups of cards of the same value, such as four sevens, with the cards they are dealt or by getting cards from other players or the deck. This give and take of cards is a simple analogy for the way atoms give and take valence electrons in chemical reactions.
What Are Valence Electrons?
Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms. Valence electrons are generally the electrons that are farthest from the nucleus. As a result, they may be attracted as much or more by the nucleus of another atom than they are by their own nucleus.
Electron Dot Diagrams
Because valence electrons are so important, atoms are often represented by simple diagrams that show only their valence electrons. These are called electron dot diagrams, and three are shown below. In this type of diagram, an element's chemical symbol is surrounded by dots that represent the valence electrons. Typically, the dots are drawn as if there is a square surrounding the element symbol with up to two dots per side. An element never has more than eight valence electrons, so there can’t be more than eight dots per atom.
Q: Carbon (C) has four valence electrons. What does an electron dot diagram for this element look like?
A: An electron dot diagram for carbon looks like this:
Valence Electrons and the Periodic Table
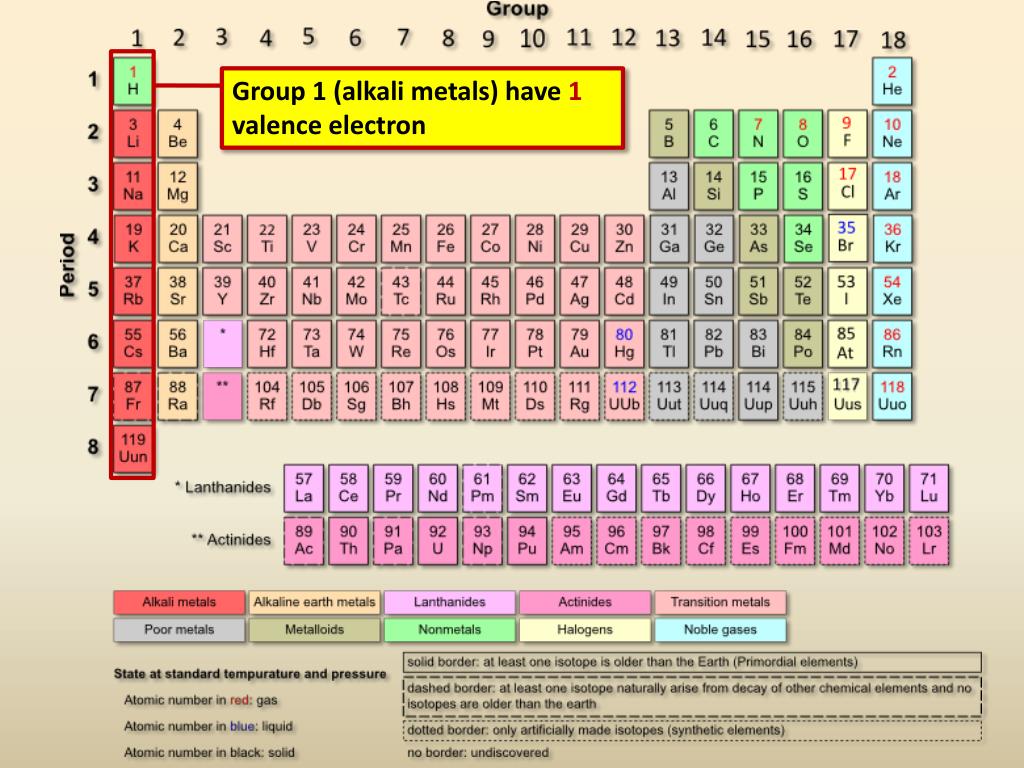
The number of valence electrons in an atom is reflected by its position in the periodic table of the elements (see the periodic table in the Figure below). Across each row, or period, of the periodic table, the number of valence electrons in groups 1–2 and 13–18 increases by one from one element to the next. Within each column, or group, of the table, all the elements have the same number of valence electrons. This explains why all the elements in the same group have very similar chemical properties.
For elements in groups 1–2 and 13–18, the number of valence electrons is easy to tell directly from the periodic table. This is illustrated in the simplified periodic table in the Figure below. It shows just the numbers of valence electrons in each of these groups. For elements in groups 3–12, determining the number of valence electrons is more complicated. You can learn more about the valence electrons of elements in these groups at this URL:http://www.colorado.edu/physics/2000/periodic_table/transition_elements.html.
Q: Based on both periodic tables above (Figures above and above), what are examples of elements that have just one valence electron? What are examples of elements that have eight valence electrons? How many valence electrons does oxygen (O) have?
A: Any element in group 1 has just one valence electron. Examples include hydrogen (H), lithium (Li), and sodium (Na). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, like all the other elements in group 16, has six valence electrons.
Valence Electrons and Reactivity
The table salt pictured in the Figure below contains two elements that are so reactive they are rarely found alone in nature. Instead, they undergo chemical reactions with other elements and form compounds. Table salt is the compound named sodium chloride (NaCl). It forms when an atom of sodium (Na) gives up an electron and an atom of chlorine (Cl) accepts it. When this happens, sodium becomes a positively charged ion (Na+), and chlorine becomes a negatively charged ion (Cl-). The two ions are attracted to each and join a matrix of interlocking sodium and chloride ions, forming a crystal of salt.
Q: Why does sodium give up an electron?
A: An atom of a group 1 element such as sodium has just one valence electron. It is “eager” to give up this electron in order to have a full outer energy level, because this will give it the most stable arrangement of electrons. You can see how this happens in the animation at the following URL and in the Figure below. Group 2 elements with two valence electrons are almost as reactive as elements in group 1 for the same reason.
Q: Why does chlorine accept the electron from sodium?
A: An atom of a group 17 element such as chlorine has seven valence electrons. It is “eager” to gain an extra electron to fill its outer energy level and gain stability. Group 16 elements with six valence electrons are almost as reactive for the same reason.
Atoms of group 18 elements have eight valence electrons (or two in the case of helium). These elements already have a full outer energy level, so they are very stable. As a result, they rarely if ever react with other elements. Elements in other groups vary in their reactivity but are generally less reactive than elements in groups 1, 2, 16, or 17.
Q: Find calcium (Ca) in the periodic table (see Figure above). Based on its position in the table, how reactive do you think calcium is? Name another element with which calcium might react.
A: Calcium is a group 2 element with two valence electrons. Therefore, it is very reactive and gives up electrons in chemical reactions. It is likely to react with an element with six valence electrons that “wants” to gain two electrons. This would be an element in group 6, such as oxygen.
Valence Electrons and Electricity
Valence electrons also determine how well—if at all—the atoms of an element conduct electricity. The copper wires in the cable in the Figure below are coated with plastic. Copper is an excellent conductor of electricity, so it is used for wires that carry electric current. Plastic contains mainly carbon, which cannot conduct electricity, so it is used as insulation on the wires.
Q: Why do copper and carbon differ in their ability to conduct electricity?
A: Atoms of metals such as copper easily give up valence electrons. Their electrons can move freely and carry electric current. You can see in detail how this occurs at the URL below. Atoms of nonmetals such as the carbon, on the other hand, hold onto their electrons. Their electrons can’t move freely and carry current.
A few elements, called metalloids, can conduct electricity, but not as well as metals. Examples include silicon and germanium in group 14. Both become better conductors at higher temperatures. These elements are called semiconductors.
Q: How many valence electrons do atoms of silicon and germanium have? What happens to their valence electrons when the atoms are exposed to an electric field?
A: Atoms of these two elements have four valence electrons. When the atoms are exposed to an electric field, the valence electrons move away from the atoms and allow current to flow.
Summary
- Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms.
- Because valence electrons are so important, atoms may be represented by electron dot diagrams that show only their valence electrons.
- The number of valence electrons in atoms may cause them to be unreactive or highly reactive. For those atoms that are reactive, the number of valence electrons also determines whether they tend to give up or gain electrons in chemical reactions.
- Metals, which easily give up electrons, can conduct electricity. Nonmetals, which attract electrons, generally cannot. Metalloids such as silicon and germanium can conduct electricity but not as well as metals.
Explore More
Watch the video at the following URL, and then answer the questions below.
http://www.youtube.com/watch?v=iuj_hw_MFN8 (9:41)
Take an Assessment below
Valence Electron
Valence Electrons
As was mentioned in a previous section of this chapter, electrons are highly important, because a specific subset of electrons, called valence electrons, are solely-responsible for determining how elements bond with one another. The number of valence electrons that are present in an atom can be determined from that atom's electron configuration. Valence electrons are found in the orbitals associated with an atom's highest occupied energy level. The remaining electrons, which are called inner shell electrons, do not participate in bonding and are, therefore, not important to study.
Consider sulfur's electron configuration, which was determined in the previous section and is replicated below.
1s22s22p63s23p4
Recall that the energy levels in an electron configuration are the leading red numbers that denote the start of a new energy level/orbital combination. Sulfur has electrons in the first, second, and third energy levels, as indicated by the leading red1, 2's, and 3's, respectively. Valence electrons are those found in the highest occupiedenergy level. Therefore, in this case, only those electrons associated with an energy level/orbital combination beginning with a 3 need to be considered. Since two energy level/orbital combinations begin with a 3, both orbitals are selected for further consideration:
3s23p4
The superscripts associated with these orbitals total to 6. Therefore, sulfur has 6 valence electrons.
Determine how many of nitrogen's electrons are classified as valence electrons. Nitrogen's electron configuration, which was determined in the previous section, is shown below.
1s22s22p3
Solution
Nitrogen has electrons in the first and second energy levels, as indicated by the leading red1 and 2's, respectively. Valence electrons are those found in the highest occupiedenergy level. Therefore, in this case, only those electrons associated with an energy level/orbital combination beginning with a 2 need to be considered. Since two energy level/orbital combinations begin with a 2, both orbitals are selected for further consideration:
2s22p3
The superscripts associated with these orbitals total to 5. Therefore, nitrogen has 5 valence electrons.
Determine how many of the electrons in each of the following elements are classified as valence electrons. Each element's electron configuration, which was determined in the previous section, is shown below.
- Neon
1s22s22p6
- Calcium
1s22s22p63s23p64s2
Valence Electrons In Nitrogen
 energy level/orbital combination beginning with a 2 need to be considered. Since two energy level/orbital combinations begin with a 2, both orbitals are selected for further consideration:
energy level/orbital combination beginning with a 2 need to be considered. Since two energy level/orbital combinations begin with a 2, both orbitals are selected for further consideration: 
2s22p6
The superscripts associated with these orbitals total to 8. Therefore, neon has 8 valence electrons. energy level/orbital combination begins with a 4, only one orbital is selected for further consideration:
energy level/orbital combination begins with a 4, only one orbital is selected for further consideration: 4s2
The superscript associated with this orbital is a 2. Therefore, calcium has 2 valence electrons.While an electron configuration represents all of the electrons present in an atom of an element, chemists are only truly interested in an atom's valence electrons, since, as indicated above, those are the electrons that are solely-responsible for determining how elements bond with one another. Therefore, finding a 'shortcut' for determining how many valence electrons are present in an atom would be highly convenient. Such a 'shortcut' does, indeed, exist. In a previous section of this chapter, three systems for labeling the groups, or columns, on the periodic table were presented. The second system, which is called the 'A/B System,' was indicated to provide insight into the electronic character of elements found within that group.
Again, consider sulfur, S, which, based on its electron configuration, has 6 valence electrons.
Sulfur is located in the 16th column of the periodic table. However, the 'A/B System' is used to label the main group elements. Group 16 is the 6th column in the main group, or 'A-Block,' columns of the periodic table and so is labeled as Group 6A. Note that sulfur's valence electron count matches its group number in the 'A/B System.' This connection applies to nearly all elements found in the main group columns of the periodic table. Helium is the only exception to this rule, as it is found in Group 8A, but only contains two total electrons. This inconsistency invalidates the 'A/B shortcut' method, and the electron configuration method must be employed to determine that both of helium's electrons are valence electrons.
Since the 'A/B System' group number corresponds to the number of valence electrons that are present in an atom, all elements found within the same column have the same number of valence electrons. Since an atom's valence electrons are solely-responsible for determining how elements bond with one another, this commonality in electronic character explains why all of the elements within the same group share similar properties.
Based on its location on the periodic table, determine how many of nitrogen's electrons are classified as valence electrons.
Solution
The 'A/B System' group number indicates the number of valence electrons that are present in an atom. Nitrogen (N) is located in the 15th column of the periodic table. However, the 'A/B System' is used to label the main group elements. Group 15 is the 5th column in the main group, or 'A-Block,' columns of the periodic table and so is labeled as Group 5A. Therefore, nitrogen has 5 valence electrons. (This answer is consistent with the solution to Example (PageIndex{1}).)
Valence Electrons In I2
Based on the periodic table, determine how many of the electrons in each of the following elements are classified as valence electrons.
- Neon
- Calcium
- Answer a
- The 'A/B System' group number indicates the number of valence electrons that are present in an atom. Neon (Ne) is located in Group 18, which is labeled as Group 8A, using the 'A/B System.' Therefore, neon has 8 valence electrons. (This answer is consistent with the solution to Exercise (PageIndex{1}text{a}).)
- Answer b
- Calcium (Ca) is located in Group 2, which is labeled as Group 2A in the 'A/B System.' Therefore, calcium has 2 valence electrons. (Again, this answer is consistent with the solution to Exercise (PageIndex{1}text{b}).)
